Dr. Zappasodi has a longstanding interest in immunosuppressive CD4+ T cells, which are critical in tumor immune evasion and immunotherapy resistance. Her work led to the discovery of a non-conventional immunosuppressive CD4+ T-cell subset with T-follicular-helper(Tfh)-like features that is specifically modulated by inhibition of the immune checkpoints PD-1 and CTLA-4. In addition, her studies revealed novel mechanisms explaining how GITR co-stimulation and CTLA-4 inhibition alter the phenotypic and functional stability of immunosuppressive regulatory T cells (Tregs). Specifically, Dr. Zappasodi was the first to describe that CTLA-4 blockade dampens the immunosuppressive function of Tregs by altering their metabolism in a way that is dependent on the glucose availability within the microenvironment.
Building on these findings, the Zappasodi lab investigates cellular and molecular mechanisms supporting dysfunctional and immunosuppressive T cells in the TME. Specifically, we study the role of tumor metabolic adaptation in disrupting tissue homeostasis, resulting in immune suppression and T-cell exclusion from the microenvironment. Additionally, we investigate how specialized CD4+ T-cell subsets, such as Tregs and Tfh, interact with B cells (as professional antigen presenting cells) and CD8+ T cells and shape the adaptive immune response to cancer. We primarily study these mechanisms in solid tumors, where the immune system and the neoplastic disease are two distinct tissue entities, and investigate similar findings in B-cell lymphomas, where the tumor arises within the immune system itself. The ultimate goal of the Zappasodi lab is to determine new effective modalities for controlling key tumor immune evasion mechanisms and overcoming immunotherapy resistance.
Conferences

We actively participate in national and international scientific meetings, including: SITC, AACR, CICON Annual Meetings
Research Projects
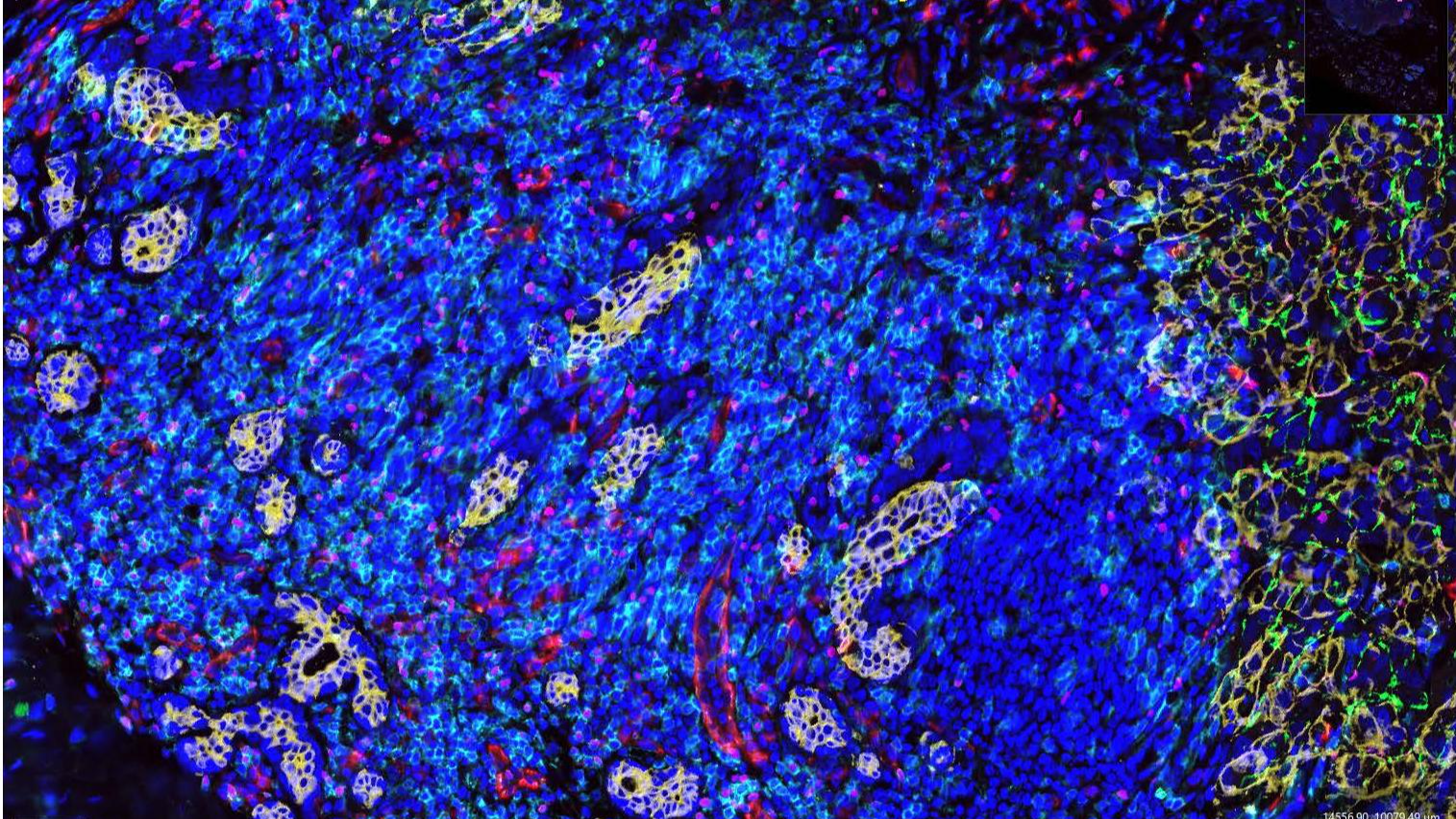
Ongoing research projects include: 1) the role of T-follicular-helper cells in the anti-tumor activity of immune checkpoint blockade therapy; 2) the impact of tumor metabolic plasticity on the tumor microenvironment and immunosuppressive T cells; and 3) the study of the immune microenvironment in the pathogenesis of B-cell lymphomas and in the response of these diseases to immunotherapy.


